

Johnson & Johnson’s vaccine is effective after only one dose, while Moderna and Pfizer both require two. What else makes the Johnson & Johnson vaccine different? Vaccine recipients cannot catch COVID-19 or a cold from any of the vaccines because they do not contain live virus.
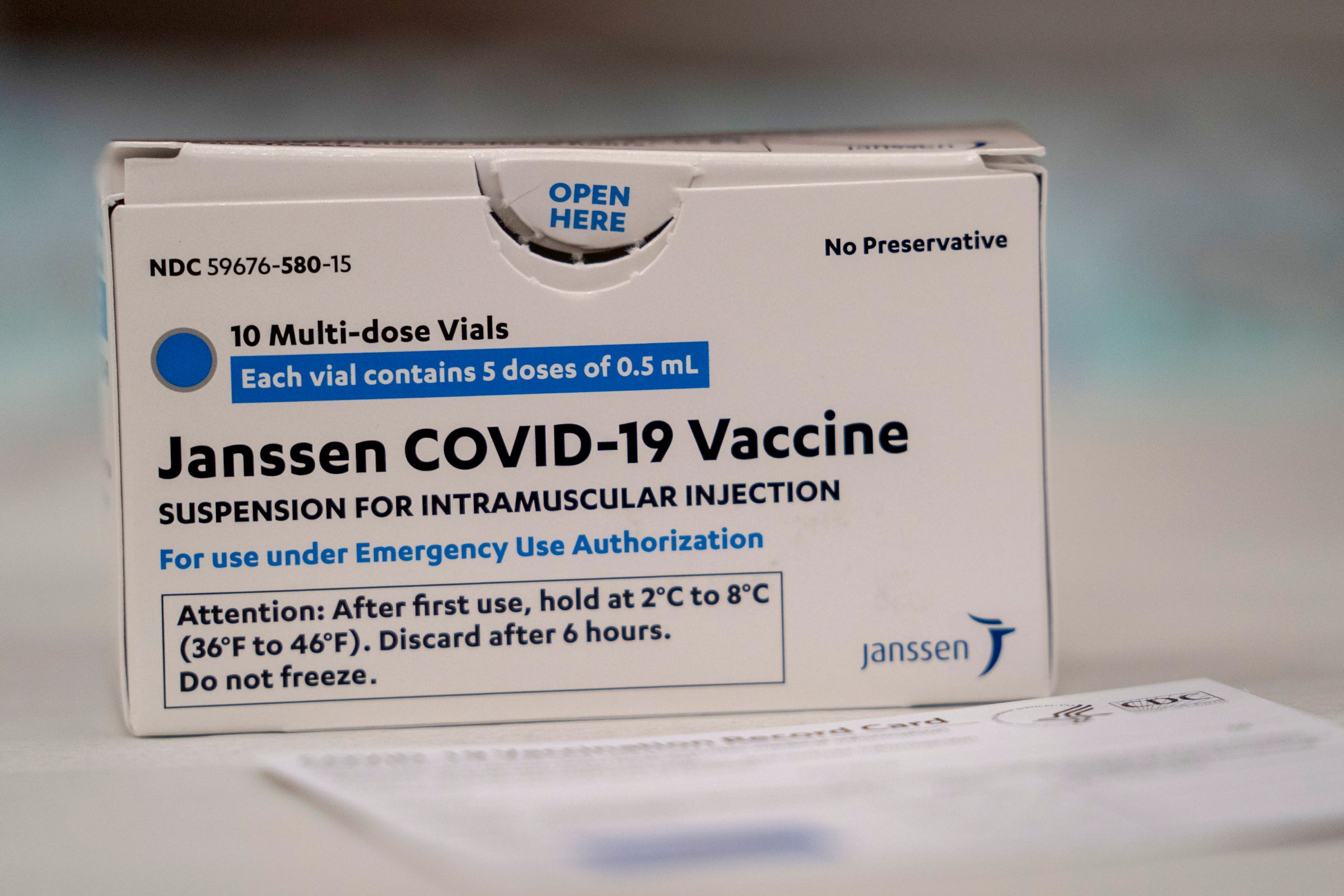
Once the cells create the spike protein found on the coronavirus, the immune system responds, conferring protection if the person is exposed to the real virus. That DNA is encapsulated in an inactivated adenovirus - the virus that causes the common cold - that cannot replicate in the body. Johnson & Johnson’s vaccine also delivers the body a recipe for creating the spike protein, but it uses DNA instead of RNA. Moderna and Pfizer’s vaccines enclose their mRNA in lipid nanoparticles, for which manufacturing facilities have had trouble ramping up production, contributing to bogged-down supply chain issues. Those two use messenger RNA to direct the body to make the virus’ spike protein. unit, is created using similar, but slightly different technology than is used in making the Pfizer and Moderna vaccines. The vaccine, produced by Johnson & Johnson’s Janssen Pharmaceutical Cos. What’s in the vaccine, and how does it work? WHYY thanks our sponsors - become a WHYY sponsorīut ultimately the FDA and CDC decided that J&J’s one-and-done vaccine is critical to fight the pandemic - and that the small clot risk could be handled with warnings to help younger women decide if they should use that shot or an alternative. Three died, and seven remain hospitalized. The government uncovered 15 vaccine recipients who developed the highly unusual kind of blood clot, out of nearly 8 million people given the J&J shot.
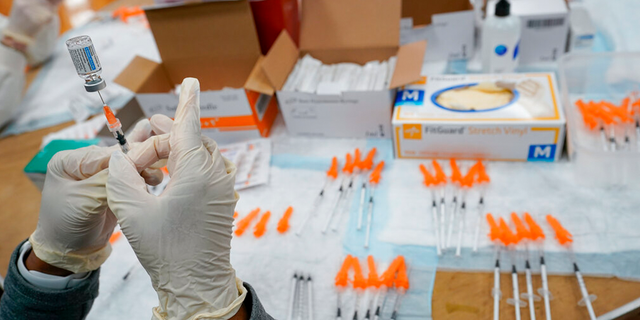
health officials lifted the pause after scientific advisers decided the vaccine’s benefits outweigh the risks. Th e FDA and the Centers for Disease Control and Prevention called April 13 for a pause in use, “in an abundance of caution,” after six women experienced a rare and severe type of blood clot after receiving the vaccine.
JOHNSON AND JOHNSON VACCINE PRODUCTION ISSUES SERIES
This is one of a series of articles in which reporters from WHYY’s Health Desk Help Desk answer questions about vaccines and COVID-19 submitted by you, our audience.ĭoses of Johnson & Johnson’s coronavirus vaccine for people ages 18 and older was first authorized for emergency use by the Food and Drug Administration in late February. Ask us about COVID-19: What questions do you have about the coronavirus and vaccines?


 0 kommentar(er)
0 kommentar(er)
